Subcutaneous Tumor Models
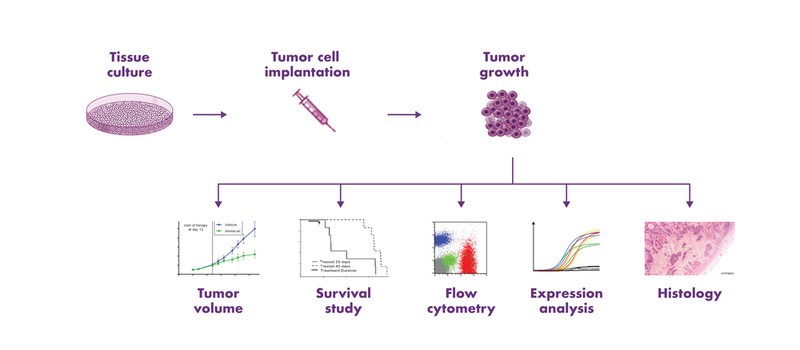
Subcutaneous tumor models are an efficient and cost-effective choice for the determination of the response of tumors to a new drug. The tumor cells are implanted into the flank of mice, and tumor growth is monitored by calipering, which enables the evaluation of anti-cancer therapies in vivo. Subcutaneous mouse models are used extensively in the scientific community, and a rich amount of historical in vivo study data are available for the characterization of these mouse models and comparison of tumor responses to other drugs. Furthermore, subcutaneous tumor models enable a variety of endpoint analyses, such as expression analysis and flow cytometry.
Subcutaneous tumor implantation into the flank of mice is minimally invasive and does not require anesthesia. Subcutaneous tumor usually form spheric structures with limited interaction with the surrounding stroma.
- Tumor models for fast and economic efficacy testing
- Workhorse models for efficacy testing of a variety of drugs in the lead optimization phase
- Xenograft and syngeneic subcutaneous tumor models are available for in vivo drug testing
- Subcutaneous tumor model study reports are written by PhD-level medical writers and custom-tailored for each project
- Meticulous documentation of drug efficacy testing following GV-Solas and ISO 9001 requirements
Reaction Biology has a global network of business development managers to understand your specific research needs and ensure your needs are met. Throughout the study, you will work with just one team of scientists to facilitate consistent communication. Should you have inquiries or if you want to directly request a quote for our subcutaneous syngeneic or xenograft models, reach out to our team today.